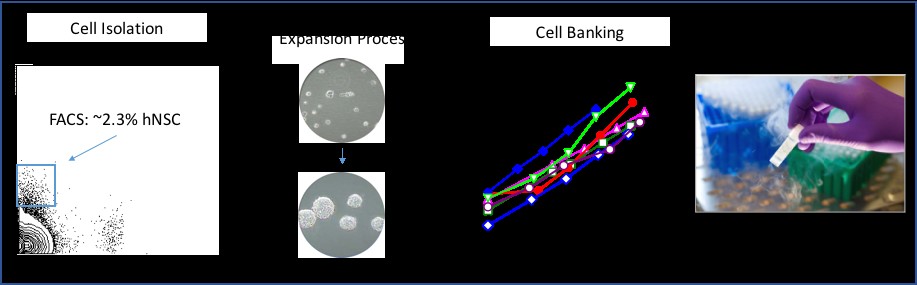
In 2000, the human neural stem cell (hNSC) was first identified and purified at the single cell level by Dr. Nobuko Uchida, Chief Scientific Officer of BOCO ReGen Med. This purified cell was shown to be true somatic (adult; non-embryonic) human neural stem cells that self renew, capable of multilineage maturation to the major cells types of the CNS; neurons, astrocytes and myelin producing oligodendrocytes. These cells are purified from human brain tissue from which a final patient dose is prepared.
The cell based regenerative medicine product, hNSC is an adult, tissue-specific stem cell, that is purified from human brain tissue using a monoclonal antibody based high-speed cell sorting process. Further purification of hNSC cells is achieved through selective passage expansion growing as neurospheres in a defined, serum free culture medium to create banks of these cells [Uchida et al. 2000] (Figure 1). Long-term expandable neurosphere cultures has allowed for reproducible generation of cryopreserved hNSC banks; each from a single brain tissue. Karyotype and morphological stability have been demonstrated with more than ten passages in long-term culture. For clinical studies, the hNSCs are manufactured by qualified personnel working in a clean room production environment, according to current Good Manufacturing Practices (cGMP). Master and Working cell banks are generated and stored as cryopreserved cell banks ready for formulation for clinical application. The cryopreserved cells are thawed and formulated for direct transplantation into the central nervous system (brain, spinal cord or retina of the eye) and shipped to the clinical site for administration by a qualified neurosurgeon or retinal surgeon. The company intends to develop the hNSC produce as an allogeneic cell therapy for specific CNS disorders based on both neuroprotective and neuronal replacement strategies starting with disorders of the retina, specifically age-related macular degeneration.
Figure 1. Overview of the hNSC isolation, expansion and banking, and Final Product.
 The biological properties of hNSC cells have been extensively characterized both in culture assays and in vivo in immunodeficient or immune suppressed animals. For potential life-long benefit to patients, we believe that robust engraftment and survival are fundamental to a cell based therapy.
The biological properties of hNSC cells have been extensively characterized both in culture assays and in vivo in immunodeficient or immune suppressed animals. For potential life-long benefit to patients, we believe that robust engraftment and survival are fundamental to a cell based therapy.
Our in vivo studies show the following characteristic of the hNSC cells and
• Long-term survival of the cells after transplantation into the brain, spinal cord, and eye
• Site appropriate migration within the CNS tissue only, no migration to ectopic site to cause potential adverse effects
• Site appropriate proliferation (i.e. self-renewal) and maturation into specific neural cell types (myelin producing oligodendrocytes in white matter; neurons in grey matter)
• No hNSC related adverse findings, tumorigenesis or other safety concerns in any of the over 3,000 animals transplanted to date, and unlike pluripotent stem cells (i.e. ES cells and iPS cells), multi-potent hNSC are not tumorigenic
• Biological function and efficacy of hNSC in animal models of a lysosomal storage disease (i.e. NCL), hypomyelination, spinal cord injury, retinal degeneration, cerebral ischemia, and Alzheimer’s disease
• Requirement for the maintenance of human cells in spinal cord injured animals to preserve restored motor function
The key attributes of neural stem cells – such as self-renewal to provide a continuous reservoir of factor-producing cells, global CNS migratory properties, and their innate ability to form new normal neurons, astrocytes or oligodendrocytes (Figure 2) formed the basis for the initiation of translational studies in select human CNS disorders. The properties of our human neural stem cell, hNSC, distinguish them from the many other cell based approaches currently being promoted in regenerative medicine and position them as attractive novel therapeutics for treating the plethora of neurodegenerative conditions.
The preclinical data and early clinical data have provided the rationale for BOCO’s acquisition of the technology and the continued studies of these cells in a spectrum of human CNS disorders.
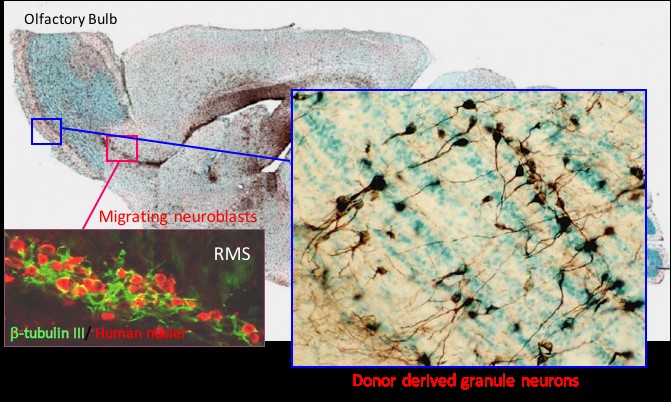
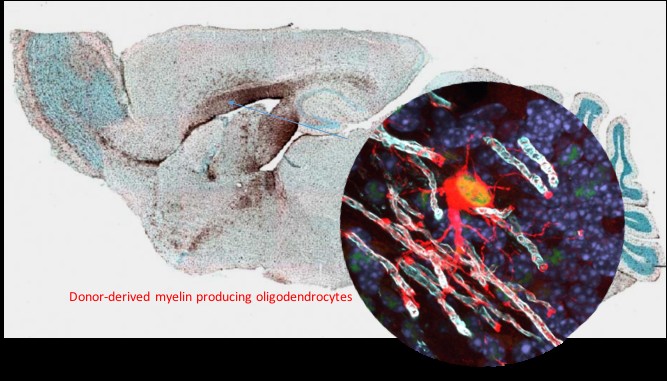
Figure 2. Long term engraftment of human NSCs in immunodeficient mouse brain.


Transplanted human cells detected as brown stained cells showing global migration throughout the mouse brain. A) migration of cells through the rostral migratory stream (RMS) and site appropriate differenatiation into neurons in the olfactory bulb. B) myelin producing human oligodendrocytes in the corpus collosum.



 The biological properties of hNSC cells have been extensively characterized both in culture assays and in vivo in immunodeficient or immune suppressed animals. For potential life-long benefit to patients, we believe that robust engraftment and survival are fundamental to a cell based therapy.
The biological properties of hNSC cells have been extensively characterized both in culture assays and in vivo in immunodeficient or immune suppressed animals. For potential life-long benefit to patients, we believe that robust engraftment and survival are fundamental to a cell based therapy.