The goal of this clinical research is to develop a safe and effective therapy for GA AMD. A successful therapy will either ultimately stabilize or slow the progression of visual loss, thereby reducing the physical, emotional, and social burdens related to this major blinding disorder. An approach that protects or rescues retinal cells represents an inherently more feasible cell transplantation strategy and arguably an equally worthy regenerative goal to that of replacement. Indeed, the theme of retinal protection can be found in the rationale for administration of growth/trophic factors, dietary supplementation with vitamin A, protection from damaging effects of light, and historical retinal transplant trials (Petrukhin, 2007; Jager, 2008). The underlying strategy of this latter approach is not to specifically replace the photoreceptor cells, but to protect and stabilize the threatened rods and cones (Milam, 1993).
A treatment strategy based on protecting photoreceptor cells by providing trophic and other physiological pathways of support is attractive for several reasons. A retinal-protective strategy does not have the complexity of approaches that require replacement, integration, and survival of a specific photoreceptor cell. It has also been shown that downstream retinal neural circuitry remains intact despite loss of photoreceptor function, so the underlying pathways for signal transmission from the retina are in place if marginal photoreceptors can be stabilized or enhanced (Bergmann, 2004; Suter, 2007). It is conceivable that the loss of the supportive function for the neural retina in GA AMD can be offset by the biological properties of an exogenous cell, such as hNSC.
hNSC cells have been shown to be effective in photoreceptor and vision rescue in a rodent model of retinal degeneration, the RCS rat. With the exception of nonhuman primates, animals do not have a structure equivalent to the macula, thus no animal model can fully recapitulate all the pathological hallmarks of AMD. However, the RCS rat does demonstrate the consequences of dysfunction in the phagocytosis pathway of the RPE cells and the resulting secondary loss of photoreceptors, which are both common pathological elements in many blinding diseases including AMD. Therefore, insights into the potential therapeutic value of an intervention can be derived from rodent models that replicate the main disease elements. The progressive photoreceptor loss in the RCS rat permits both histological and behavioral assessments of experimental neuroprotective treatments that may also affect the disease course in AMD and other blinding diseases, all of which share photoreceptor death as the final common pathway.
Upon transplantation into the subretinal space at postnatal day 21 (P21) in the RCS rat model, hNSC cells protect host photoreceptors and preserve visual function. Cone photoreceptor density was found to remain constant over time, consistent with the sustained visual acuity and luminance sensitivity functional outcomes. hNSC cell behavior and fate have also illustrated the following: 1) radial migration from the injection site in the subretinal space, 2) survival throughout the seven-month experiment with maintenance of an immature phenotype at the latest time point, and 3) modest cell division, with no evidence of uncontrolled growth or tumor-like formation.
A neuroprotective strategy to prevent progression of vision loss relies upon the preservation of existing photoreceptors at a time prior to irreversible damage. In the RCS rat model, P21 correlates to a time point at which maximal protection of photoreceptor and preservation of vision can be assessed. Hence, P21 transplantation in the RCS rat was chosen to assess efficacy based on previous studies showing that even though rod number and morphology in the P21 rats are virtually indistinguishable from age-matched normal rats, rod function by physiological recording (electroretinogram [ERG]) is already severely compromised. Time points beyond P21 in this model are associated with rapid and progressive photoreceptor loss to the degree that the outcome of protective strategies cannot be as reliably measured. Thus, hNSC transplantation at the P21 stage of photoreceptor degeneration will support clinical evaluation across a broad spectrum of disease severity in humans including early stages of vision pathology.
The collective preclinical data strongly suggest that subretinal hNSC transplantation may result in the preservation of host photoreceptors by adopting one or more of the underlying RPE functions critical for maintenance of vision including phagocytosis of outer segments and delivery of soluble neurotrophic factors, both of which are deficient in deteriorating RPE. Given these findings, hNSC cells appear to be well suited for cell-based therapy in GA AMD.
Since RPE loss is neither global nor complete in GA AMD, particularly in earlier stages, sufficient residual RPE may exist for support of the visual cycling pathway (conversion of all trans-retinol to 11-cis-retinal). A cone-specific visual cycle involving the Müller cell has also been recently identified and could serve as an important pathway for cone chromophore cycling independent of RPE status (Wang, 2011). Thus, it seems reasonable that donor cells need not express the entire spectrum of RPE activity to exert a clinical benefit on vision and it is also possible that donor cells could have a direct or an indirect beneficial effect on the host RPE that also contributes to protection of photoreceptor integrity (i.e., antioxidant or immunomodulatory activity).
The natural history of GA AMD has been studied and the range of visual decline based on BCVA is generally known (Suness, 1999; Suness, 2007). The onset of GA in the setting of AMD represents a disease stage in which the subsequent clinical progression of visual deterioration can be predicted, but sufficient photoreceptors, visual function, and RPE remain amenable to rescue (Suness, 2007). Transplantation of hNSC cells into the subretinal space could lead to neuroprotection of vulnerable photoreceptors and produce a therapeutic effect over a measurable portion of the macula and fovea.

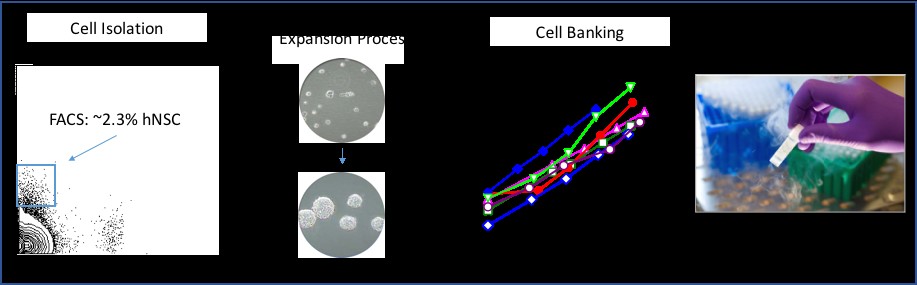
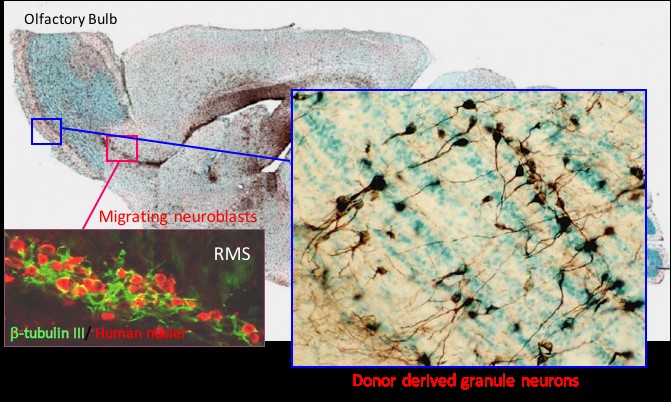
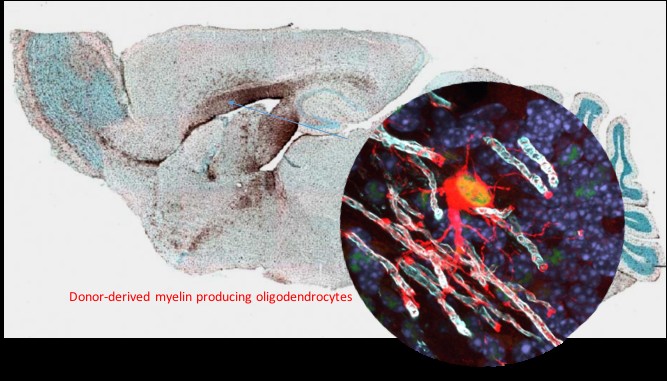 The biological properties of hNSC cells have been extensively characterized both in culture assays and in vivo in immunodeficient or immune suppressed animals. For potential life-long benefit to patients, we believe that robust engraftment and survival are fundamental to a cell based therapy.
The biological properties of hNSC cells have been extensively characterized both in culture assays and in vivo in immunodeficient or immune suppressed animals. For potential life-long benefit to patients, we believe that robust engraftment and survival are fundamental to a cell based therapy.